We will discuss on SO2 lewis structure, hybridisation and molecular geometry in this article. Here, learn about dipole moment in Sulphur dioxide bond angle and formal charges in SO2.
SO2 Lewis structure
In this structure, less electronegative sulphur atom is present as the central atom. Oxygen atoms are more electronegative so present at the corners.
Steps to make SO2 lewis structure
- Count the total valence electrons in SO2 molecule.
- S has 6 and each O atom has 6 valence electrons. So, total valence electrons in SO2 molecule are 6 + 2*6 = 18 valence electrons.
- Firstly place bond pair between sulphur and oxygen thus, making 4 valence electrons used up.
- Now, complete the octet of O atoms i.e. 3 lone pairs or 6 valence electrons around O atoms.
- Remaining lone pair or 2 valence electrons place them on S atom.
- O atoms have completed their octet but S has not.
- So, move one lone pair from O atom to S-O bond pair making it S=O double bond.
- Now, the octet of each atom is completed.
O
\
// S: SO2 Sulphur dioxide molecule Lewis structure
O 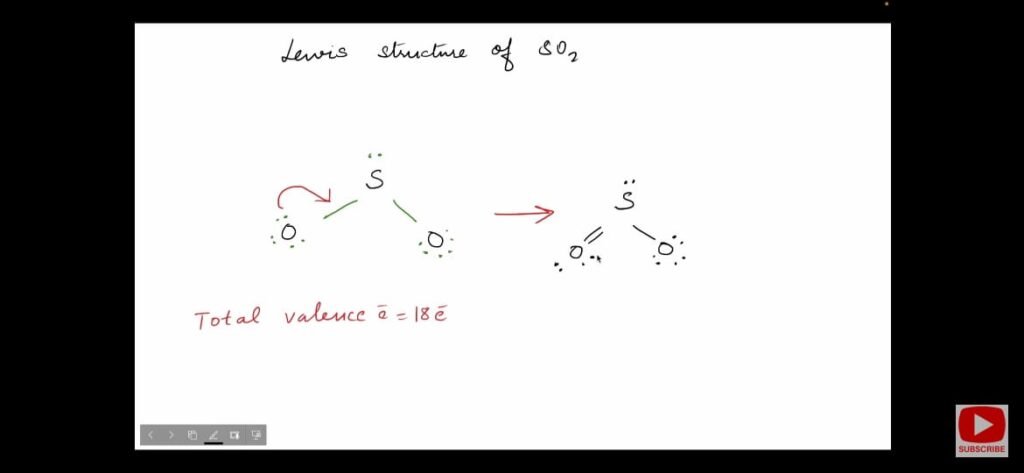
Go to the video to understand the concept step by step ;
How to make lewis structure of SO2
Formal charge calculation in SO2 molecule
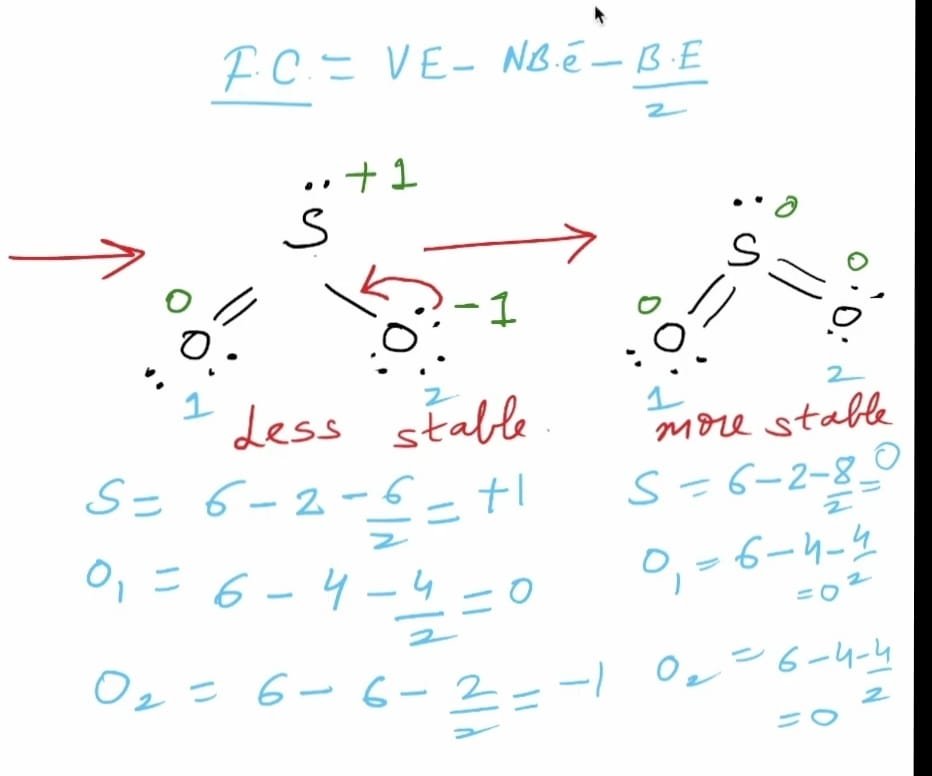
Formal charge = number of valence electrons – number of lone pair electrons – 1/2(number of bond pair electrons)
F.C. on Sulphur atom
Valence electrons of sulphur = 6
Bond Pair electrons = 6
Non-bonding electrons = 2 electrons ( 1 L.P. )
Formal charge = 6 -2 -6/2 = +1
F.C. on first oxygen atom
Valence electrons of oxygen = 6
Bond Pair electrons = 1 double bond = 4 electrons
Non-bonding electrons = 4 electrons ( 2 L.P. )
Formal charge = 6-4-4/2 = 0
F.C. on second oxygen atom
Valence electrons of oxygen = 6
Bond Pair electrons = 1 single bond = 2 electrons
Non-bonding electrons = 6 electrons ( 3 L.P. )
Formal charge = 6-6-2/2 = -1
The formal charges on the sulphur (S) atom is +1 and on the second oxygen (O) atom is -1 which indicates that the Lewis structure obtained of SO2 is not stable yet.
As sulphur can expand its octet so, SO2 will show more stable structure
Formal charge = number of valence electrons – number of lone pair electrons – 1/2(number of shared electrons)
F.C. on sulphur (S) = 6 – 2 – 1/2(8) = 0
The formal charge on each oxygen atom can be calculated using the same formula as above
F.C. on each oxygen atom = 6 – 2 – 1/2(4) = 0
Therefore, in the Lewis structure of SO2, the formal charges on all the atoms add up to zero to make it a neutral molecule.
Hybridisation of SO2
The hybridisation of Sulphur atom in SO2 is Sp2.
To determine the hybridisation of SO2 count the total number of electron pairs around S atom. There are two double bonds and one lone pair of electrons around the sulphur atom.
In sp2 hybridisation, the 3s orbital and two of the three 3p orbitals of sulphur combine to form 3 sp2 hybrid orbitals that are oriented in a trigonal planar geometry. The remaining p orbital on sulphur overlaps with a p orbital on each oxygen atom to form 2 additional pi bonds. Therefore, in SO2, the sulphur atom is Sp2 hybridised that allows it to form 2 double bonds and one lone pair with the 2 oxygen atoms.
Shape of SO2
The shape of SO2 is bent or V-shaped.
The 2 double bonds and the lone pair of electrons on the sulphur atom give rise to a bent molecular geometry or V-shaped geometry. The bond angle between 2 oxygen atoms and the sulphur atom is nearly 119 degrees, which is less than the ideal tetrahedral angle of 109.5 degrees. According to VSEPR, lone pair of electrons on the sulphur atom repels the bonding electron pairs and causes them to move closer together.
FAQs on SO2
Q: What is the Lewis structure of SO2?
A: The Lewis structure of SO2 consists of one S atom double-bonded to 2 oxygen atoms and one lone pair of electrons on the sulphur atom.
Q: What is the hybridisation of the sulphur atom in SO2?
A: The sulphur atom in SO2 undergoes Sp2 hybridisation. One 3s orbital and two of the three 3p orbitals combine to form 3 Sp2 hybrid orbitals. Sp2 hybridised orbitals are oriented in a trigonal planar geometry.
Q: What is the bond angle in SO2?
A: The bond angle in SO2 is approximately 119 degrees.
Q: Why is the bond angle in SO2 less than 120 degrees ?
A: The bond angle in SO2 is less than 120 degrees due to the repulsion between the bond pairs and the lone pair of electrons on the sulphur atom according to VSEPR
What is the structure of NH3
Check out the structure of NH3 here
For more updates join me on 👇
You Tube Channel Chemistry with mona mam
Website Basics of chemistry.comInstagram
