Here in this article we will study about 03 Lewis structure formal charge. We will also discuss 03 Lewis structure, 03 resonance, O3 dipole moment and O3 molecular geometry. Here, you will know about the O3 ozone bond angle and O3 lewis structure formal charge calculation.
O3 Lewis Structure
The Lewis structure of O3 consists of 3 oxygen atoms. Each oxygen atom has six valence electrons. Total number of valence electrons in O3 are 18 (6 x 3).
Draw the Lewis structure of O3 using these steps:
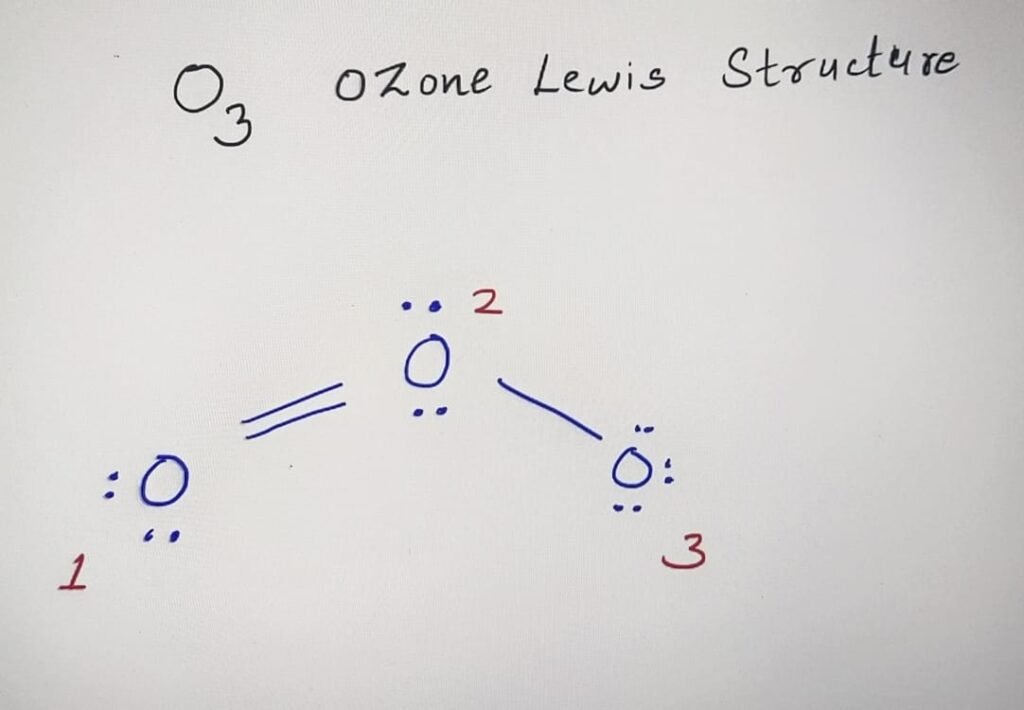
1 : We can consider any oxygen atom as the central atom in O3 Ozone molecule.
2 : Total number of valence electrons are 18 in Ozone molecule.
3 : Firstly connect all the oxygen atoms using single bonds.
4 : Remaining electrons are distributed around the O atoms to make their octet complete. The oxygen atoms at the corners will have 3 and 2 lone pairs. Central Oxygen atom carries 2 Lone pairs of electrons.
The Lewis structure of O3 ozone :
O3 Lewis structure Formal charge
Here’s how to calculate the formal charges for each atom in O3:
Formal charge on Oxygen Atom Number 2 :
Valence electrons in O atom – number of lone pair electrons – 1/2 x number of bonding electrons
= 6 – 2- 1/2 x 6
= +1
Formal charge on Oxygen Atom Number 1 :
Valence electrons in O atom Number 1 = number of lone pair electrons – 1/2 x number of bonding electrons
= 6 – 4 – 1/2 x 4
= 0
Formal charge on Oxygen Atom Number 3 :
Valence electrons in O atom Number 3 = number of lone pair electrons – 1/2 x number of bonding electrons
= 6 – 6 – 1/2 x 2
= -1

Resonating structures of O3 Lewis structure
Theoretically, in the first resonating structure double bond is present between the central oxygen atom and one of the corner oxygen atoms. And in the second resonating structure, the double bond is present between the central oxygen atom and the other corner oxygen atom.
Autually, the molecule is a hybrid of all 3 structures, with the electrons delocalised over all 3 oxygen atoms, resulting in a resonance hybrid.

Bonding and molecular geometry in O3 Ozone
Lewis structure of O3 ozone has bent shape with bond angles nearly of 117 degrees. The 2 lone pairs of electrons on the central oxygen atom push the bonding pairs closer resulting in a bent shape of O3 molecule. Oxygen-oxygen bond length in O3 ozone is around 127.2 pm.
Hybridisation of O3 Lewis structure
Hybridisation of the central oxygen atom in O3 is Sp2. As central oxygen atom has 3 orbitals in the same plane arranged in a trigonal planar geometry. 3 oxygen atoms in O3 are bonded through sigma bonds and pi bonds.
Here is the complete chapter of chemical bonding
Visit the video to understand the concept of O3 Lewis structure
FAQs of O3 Ozone
Q: What is the molecular geometry of O3?
A: The molecular geometry of O3 is bent or V-shaped.
Q: What is the hybridisation of the central oxygen atom in O3?
A: The hybridisation of the central oxygen atom in O3 is Sp2.
Q: What is the bond angle in O3?
A: The bond angle in O3 is approximately 117 degrees.
Q: What is the bond order of the O-O bonds in O3?
A: The O-O bonds in O3 is in between single bonds and double bonds, so their bond order is approximately 1.5.
Q: What is the total number of valence electrons in O3?
A: The total number of valence electrons in O3 is 18 (6 from each oxygen atom).
Q: Can O3 act as an oxidising agent?
A: Yes, O3 can act as an oxidising agent due to the presence of its highly reactive oxygen atoms. O3 can oxidize other substances by releasing oxygen atoms to them.
Q: How is O3 formed in the atmosphere?
A: O3 is formed in the atmosphere through a series of chemical reactions involving sunlight and pollutants such as nitrogen oxides and volatile organic compounds.
Check out the structure of NH3 here
For more updates join me on
You Tube Channel Chemistry with mona mam
Website Basics of chemistry.comInstagram

Nice
thank you