Here, you will learn about H2O lewis structure molecular geometry. You will also study about H2O structure and bond angle. You will come to know here about the formal charges in H2O lewis structure.
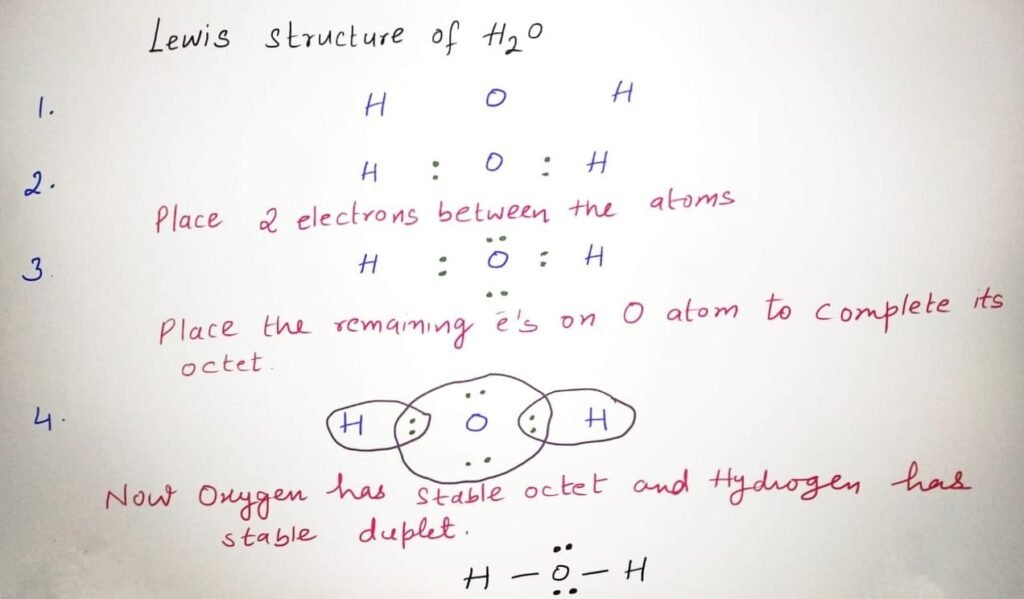
How to draw H2O lewis structure
The Lewis structure of H2O, or water, can be drawn by following these steps:
Calculate the total number of valence electrons in the molecule. For H2O, this would be 2 + 6 = 8, since hydrogen has 1 valence electron and oxygen has 6.
Place the least electronegative atom Hydrogen in the centre of the structure. Oxygen will be placed on either side of hydrogen.
Connect each atom to the central atom with a single bond, which represents a pair of shared electrons. The oxygen atoms will each have two non-bonding pairs of electrons or lone pairs.
Place the remaining valence electrons as lone pairs on the oxygen atoms to complete their octet.
Formal charges in H2O Lewis structure
Formal charge = valence electrons – non-bonded electrons – 1/2 * number of bonded electrons
Using this formula, we can calculate the formal charges for the H2O molecule as follows:
For the Oxygen atom:
F.C. calculation = 6 – 4 – 1/2 * 4 = 0
For each Hydrogen atom:
F.C. calculation = 1 – 0 – 1/2 * 2 = 0
Therefore, in the H2O molecule, the formal charges on both hydrogen atoms and oxygen atom are zero. It indicates that water molecule is neutral. It does not have any significant charge imbalance.
Hybridisation in Lewis structure of H2O
The hybridisation of the water molecule is Sp3. This means that the oxygen atom in water has four hybrid orbitals. The four hybrid orbitals are arranged tetrahedrally around the oxygen atom.
The two lone pairs of electrons on the oxygen atom occupy two of the hybrid orbitals. While the other two hybrid orbitals form 2 O-H covalent bonds. The hybridisation of the oxygen atom allows it to form covalent bonds with the 2 hydrogen atoms in a tetrahedral geometry. This gives the water molecule its V-shaped or bent structure.
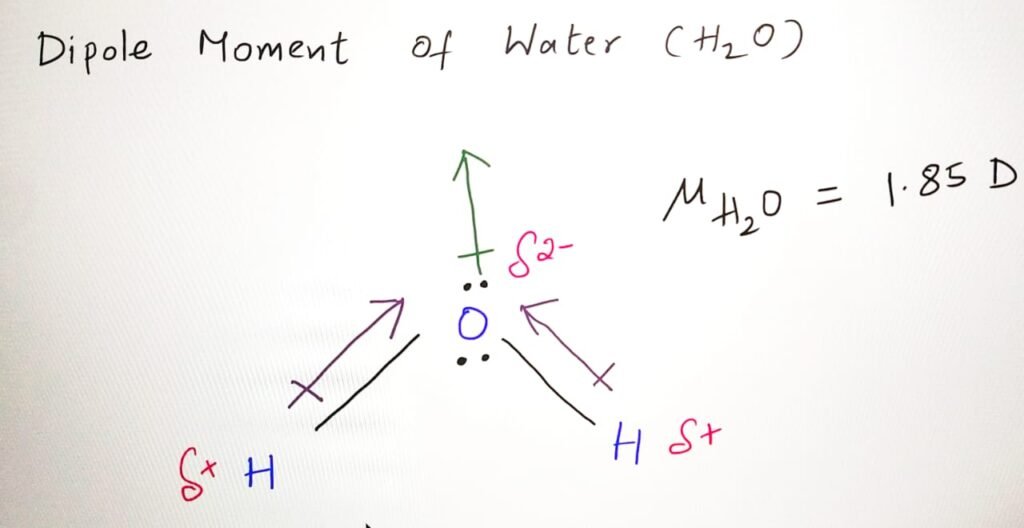
Dipole moment of H2O Lewis structure
Water (H2O) molecule has a dipole moment because the oxygen atom has a higher electronegativity than the hydrogen atoms. Electronegativity difference causes the electrons in the O-H bonds to be pulled towards the oxygen atom. This creates partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atoms.
The dipole moment of water is 1.85 Debye which is high, indicating that water is a polar molecule. The direction of the dipole moment is from the oxygen atom towards the two hydrogen atoms.
High dielectric constant of water
Water has a high dielectric constant due to its polar nature. This arises due to the separation of charges within the molecule. The high dielectric constant allows the water molecule to dissolve many ionic and polar substances.
This makes water an excellent solvent for a wide range of chemical reactions and biological processes. Also used for the separation and analysis of charged particles in electrophoresis and other separation techniques.
What Properties makes water unique molecule
Polarity of H2O molecule:
Water is a polar molecule means it has a partial positive charge on one end and a partial negative charge on the other end. Therefore, it can form hydrogen bonds with other polar molecules, which contributes to its unique properties.
High heat capacityOf H2O :
Water has a high heat capacity, which means it can absorb and store large amounts of heat energy without a significant increase in temperature. This property is important for regulating the temperature of living organisms and the environment.
High boiling and freezing points of H2O :
Water has a high boiling and freezing point compared to other molecules of similar size. High boiling and melting property of H2O allows it to form hydrogen bonds. This property is important for life as it allows water to exist as a liquid over a wide range of temperatures.
H2O as Universal solvent :
Water is often referred to as the “universal solvent” because it can dissolve a wide range of substances, including salts, sugars, and gases. This property is due to its polarity and ability to form hydrogen bonds with other polar molecules.
High surface tension of H2O :
Water has a high surface tension, which is due to the cohesive forces between water molecules. This property is important for many biological processes. It helps in the movement of water through plant roots and the surface tension of water on the surface of insects.
FAQs related to H2O lewis structure
- What is the shape or geometry of the water molecule?
- Water molecule has bent or V-shaped geometry with the oxygen atom at the centre and two H atoms bonded to it. The bond angle between the hydrogen atoms and the oxygen atom is about 104.5 degrees.
- Why does water molecule has a bent shape?
- The bent shape of the water molecule is due to the presence of 2 lone pairs of electrons on the oxygen atom. These lone pairs repel the hydrogen atoms. This causes the molecule to adopt a bent or V-shaped geometry.
- Is water molecule polar or non polar?
- The water molecule is polar due to the electronegativity difference between the oxygen and hydrogen atoms. The oxygen atom has a partial negative charge. The hydrogen atoms carries the partial positive charges.
- What is the hybridisation of oxygen atom in water?
- The oxygen atom in water has Sp3 hybridisation. This means that it has four hybrid orbitals arranged tetrahedrally around it.
- What is the dipole moment of water molecule?
- The dipole moment of the water molecule is 1.85 Debye, which indicates its polarity and ability to form hydrogen bonds.
- What is the bond angle of water molecule?
- The bond angle of the water molecule is approximately 104.5 degrees.
Check out the structure of NH3 here
For more updates join me on
You Tube Channel Chemistry with mona mam
Website Basics of chemistry.com

You made it very clear and simple to understand!