Ozone O3 preparation properties structure and uses p block elements class12
Here in this article you will study about ozone 03 preparation, properties, structure and uses P block elements class 12. You will understand the topic of ozone or three preparation properties structure and uses P block elements class 12 with the help of PDF notes and video tutorial
Notes on ozone O3 preparation properties structure and uses p block elements class12

Here is the explanation of the notes ozone O3 preparation properties structure and uses p block elements class12 in the video 👇
Preparation properties structure and uses of ozone O3 p block elements class 12
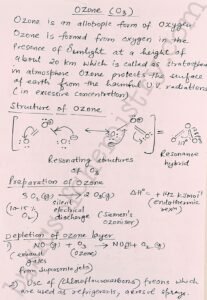
Ozone is an allotropic form of oxygen which is formed from oxygen in the presence of sunlight at a height of about 20 km in stratosphere. Ozone protects the Earth surface from the harmful UV radiations.
Preparation of ozone O3 P block elements
Ozone can be prepared by subjecting pure and dry oxygen through a silent electrical discharge in Siemens ozoniser. Formation of ozone from oxygen is an endothermic reaction. So it is essential to to do the reaction giving a silent electrical discharge in order to prevent its decomposition.
Book Now For Online Chemistry Classes
Depletion of ozone layer
The Depletion of ozone layer is occurring due to the release of oxides of Nitrogen into the stratosphere from the exhaust systems of supersonic jets.
Also the use of freons that is chlorofluorocarbons which are used as refrigerant and also in aerosol sprays are the another cause of depletion of ozone layer.
Properties of ozone O3 P block elements
Ozone is a pale blue colour as a gas, it is dark blue in the form of liquid and violet black when it is solid having a strong characteristic smell. Ozone is diamagnetic in nature and like oxygen molecule.
The Ozone is poisonous in nature and is used as a disinfectant ozone causes headache and nausea when inhaled even in small amounts.
Ozone is a good oxidising agent so, oxidises iodide ions to iodine and lead sulphide to lead sulphate.
The ozone is thermodynamically less stable than oxygen because its decomposition into oxygen results in ∆ H negative ∆ S positive. These two factors reinforce each other which results in large ∆G negative value.
Ozone is used in quantitative method for estimating ozone gas.
Resonating structures of ozone O3 P block elements
Oxygen oxygen bond length in ozone molecule is 128 pm and the bond angle is about 117 degrees the resonating structures are given above in the notes.
Uses of ozone O3 p block elements
Ozone is a very strong oxidising agent that is used in the manufacturing of KMnO4
It is used as a germicide disinfectant for sterilising water and also as a bleaching agent.
Practice questions on ozone O3 p block elements (FAQs)
1. Why does ozone acts as a powerful oxidising agent ?
2. How the supersonic jet aeroplanes are responsible for the depletion of ozone layer ?
3. Which aerosols deplete ozone ?
4. How would you account for the following :
The two oxygen – oxygen bond lengths in oxygen molecule are equal.
For more updates join me on 👇
Website – https://basicsofchemistry.com/
Instagram – https://www.instagram.com/monabindalgupta/
Facebook – https://www.facebook.com/monabindalgupta/
Twitter – https://twitter.com/monabindalgupta
Quora – https://www.quora.com/profile/Mona-Gupta-202
LinkedIn – https://www.linkedin.com/in/mona-gupta-7b450519a
