group 15 Elements p block elements class 12
Here you will learn about the oxo acids of phosphorus group 15 elements P block elements class 12. You will understand the topic of oxoacids of phosphorus group 15 elements P block elements class 12 with the help of notes and video of tutorials
Notes on group 15 Elements p block elements class 12

Oxoacids of Phosphorus p block Elements class 12 NCERT
Structure and properties of Oxoacids of Phosphorus
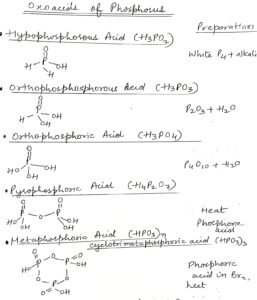
Hypophosphorous acid H3PO2
The hypophosphorous acid is prepared by the reaction of white phosphorus with alkali. Hypophosphorous is a very good reducing agent.This Hypophosphorous acid is a monobasic acid because of the presence of one replaceable hydrogen. In hypophosphorous acid oxidation state of phosphorus atom is +1
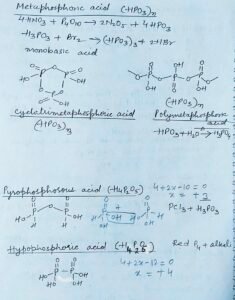
Hypophosphoric acid H4P2O6
The Hypophosphoric acid is prepared by the reaction of red phosphorus with alkali. In hypophosphoric acid the oxidation state of phosphorus is + 4
Orthophosphoric acid H3PO4
An Orthophosphoric acid is prepared by dissolving P4O10 in water. Orthophosphoric acid can be prepared by the reaction of phosphates with sulphuric acid. The ortho phosphoric acid is a tribasic acid and oxidation state of phosphorus in orthophosphoric acid is + 5
Orthophosphorous acid H3PO3
An Orthophosphorous acid is prepared by dissolving P2O3 in water. Orthophosphoric acid is a dibasic acid because of the two replaceable hydrogen atoms. In orthophosphoric acid the oxidation state of phosphorus is + 3. So orthophosphorous acid generally undergoes disproportionation reaction.
Pyrophosphorous acid H4P2O5 Oxoacids of phosphorus
The Pyrophosphorous acid can be prepared by the reaction of phosphorus trichloride and orthophosphoric acid. The oxidation state of phosphorus in pyrophosphorous acid is +3
Pyrophosphoric acid H4P2O7 Oxoacids of phosphorus
This Pyrophosphoric acid can be prepared by heating two molecules of phosphoric acid. In pyrophosphoric acid the oxidation state of phosphorus is + 5
Metaphosphoric acid
The Metaphosphoric acid is prepared by the dehydration of nitric acid with P4O10. Metaphosphoric acid also forms trimer and polymer as follows.
Cyclotrimetaphosphoric acid
Polymetaphosphoricacid
In the you tube channel video you can understand the trick to make oxoacids of Phosphorus.
Book Now For Free Online Chemistry Classes
Properties of oxoacids of Phosphorus
In all the oxyacids they can be easily prepared by the loss or gain of water molecule or oxygen atom.
All the oxyacids contain at least one P=O and one P -OH bond.
In the oxoacids hydrogen atom of OH group is ionizable and makes the oxo acid showing basicity. Hydrogen atom directly bonded to Phosphorus atom is non ionisable.
The acids which contain P-H bond have strong reducing properties. In hypophosphorous acid it is acting as a good reducing agent because of 2 P-H bonds.
Oxoacids of phosphorus are very interesting to study with their formula, methods of preparation and their characteristic bonds.
Conclusion
Here you have learnt about the structure and properties of oxoacids of phosphorus group 16 elements P block elements class 12 with the help of videos notes and explanation given above