Dioxygen O2 group 16 elements p block elements class 12
Here in this article we will learn about the preparation, properties and uses of Dioxygen (O2) group 16 elements P block elements class 12. You will understand the complete topic with the help of notes and video given below
Notes on Dioxygen O2 group 16 elements p block elements class 12
Here, is the explanation of the notes 👇 from this Youtube video
Dioxygen O2 group 16 elements p block elements class 12
Oxygen exist as a diatomic gas in nature and is called as Dioxygen. Here, are some of the important preparation, properties and uses of Dioxygen :
Preparation of Dioxygen O2 group 16 elements p block elements class 12
Di oxygen is prepared by the decomposition of oxygen rich compounds like potassium permanganate, potassium nitrate and potassium chlorate.
O2 can also be prepared by the thermal decomposition of the metal oxides which are lying low in the electrochemical series and also from the higher oxides of some metals like the examples we have mentioned above in the notes.
Di oxygen can also be prepared by the decomposition of hydrogen peroxide into water with the help of a catalyst such as finely divided metals and magnesium dioxide.
O2 can also be manufactured from water or Air on large scale. Dioxygen can also be prepared from the electrolysis of water. The products formed are hydrogen gas at cathode and Oxygen gas at the anode.
Industrially Di oxygen is obtained by the liquefaction of air.
Properties of Dioxygen O2 group 16 elements p block elements class 12
Di oxygen is a colourless and odourless gas which liquifies at 90 K and freezes at 55 k. Oxygen is generally neutral towards litmus.
Molecular oxygen is paramagnetic because of the presence of two unpaired electrons. The paramagnetic behaviour allows the oxygen molecule to get slightly attracted towards the magnetic field.
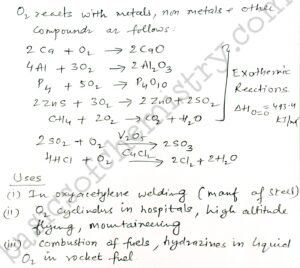
Di oxygen directly reacts with nearly all metals and nonmetals except silver, gold, platinum and noble gases.
Most of the reactions of Di oxygen are exothermic in nature. some external heating. We need some heat energy to initiate the reaction. Because the bond dissociation enthalpy of oxygen oxygen double bond is quite high.
Uses of Dioxygen O2 group 16 elements p block elements class 12
Most important use of O2 Di oxygen as oxygen cylinders in hospitals. This is widely seen all over the world in covid days.
Also O2 cylinders are used at high altitudes for flying and in mountaineering to to help in breathing.
Di oxygen is used in oxyacetylene welding for the manufacturing of different metals like Steel products.
O2 is used in the combustion of fuels. For example hydrazine in liquid oxygen acts as an important rocket fuel which provides tremendous thrust for the launch of rockets.
Important Practice Questions (FAQs) Dioxygen O2 group 16 elements p block elements class 12
1. Which of the following does not react with oxygen directly ? Zn , Ti , Pt , Fe
2. Complete the following reactions :
C2H4 +O2 =
4Al + 3 O2 =
For more latest updates join me on 👇
Website – https://basicsofchemistry.com/
Instagram – https://www.instagram.com/monabindalgupta/
Facebook – https://www.facebook.com/monabindalgupta/
Twitter – https://twitter.com/monabindalgupta
Quora – https://www.quora.com/profile/Mona-Gupta-202
LinkedIn – https://www.linkedin.com/in/mona-gupta-7b450519a