15 Important NCERT Questions Group 15 Elements P Block Elements Class 12
Notes on Important NCERT Questions Group 15 Elements P Block Elements
Here’s the explanation of the notes in the video👇
Important NCERT questions group 15 elements P block elements
• Pentahalides are more covalent than trihalides of group 15 elements
Elements of group 15 have five electrons in their valence shell. Elements in the + 5 oxidation state have less tendency to lose electrons than in + 3 oxidation State therefore, elements in + 5 oxidation State have more tendency to form covalent compounds.
• Why is BiH3 the strongest reducing agent among all the hydrides of group 15 elements ?
As we move down the group the size of the element increases and the strength of element hydrogen bond weakens. Therefore, hydrogen gas can be easily evolved from Bismuth hydride hence, Bismuth hydride is the strongest reducing agent in group 15 elements.
• Why is N2 less reactive at room temperature ?
Due to the presence of triple bond between two nitrogen atoms the bond dissociation energy of N2 is very high therefore, N2 is less reactive at room temperature.
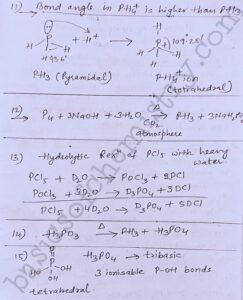
• Bond angle in PH4+ is higher than that in PH3 why ?
In PH3 Phosphorus is SP3 hybridised. PH3 has 3 bond pairs and 1 lone pair, due to inter electronic repulsions according to VSEPR theory in the molecule the normal tetrahedral angle decreases from 109.28 to 93.6
• What is the basicity of H3PO4 ?
In the structure of H3PO4 the molecule is tetrahedral. H3PO4 has 3 ionizable P – H bonds therefore as H3PO4 is tribasic in nature.
For more updates join me on 👇
Website – https://basicsofchemistry.com/
Instagram – https://www.instagram.com/monabindalgupta/
Facebook – https://www.facebook.com/monabindalgupta/
Twitter – https://twitter.com/monabindalgupta
Quora – https://www.quora.com/profile/Mona-Gupta-202
LinkedIn – https://www.linkedin.com/in/mona-gupta-7b450519a